Prime Editing Advances the Accuracy of Cancer Mouse Models
Scientists can now effectively study any cancer mutation they wish.
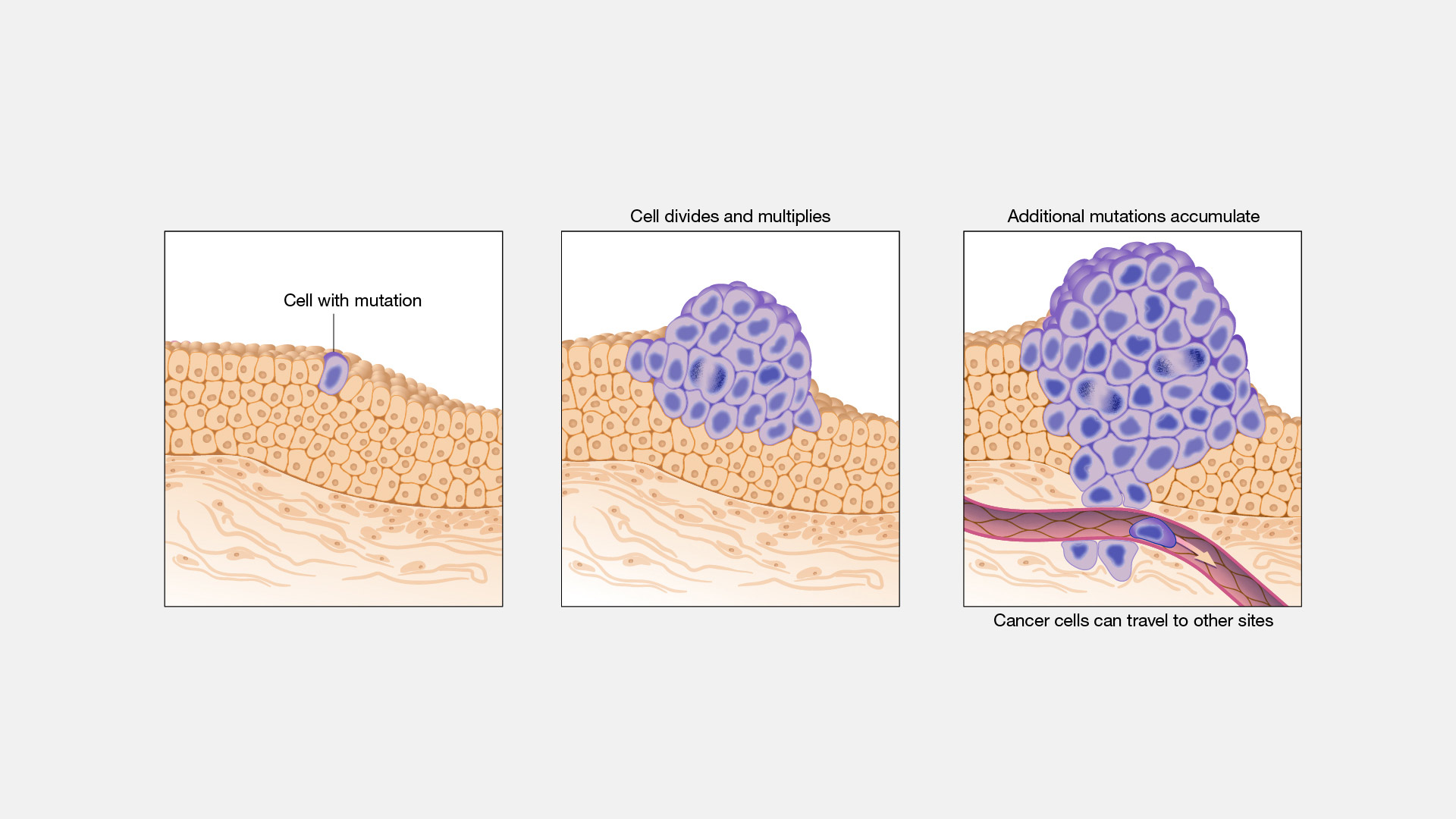
Cancer arises from a series of genetic mutations in our cells that disrupt normal cellular functions. These mutations can be triggered by various factors, including environmental influences, lifestyle choices, and inherited genetic predispositions. When important genes that control cell growth get damaged, cells can start growing out of control and form tumors. In fact, genomic research on cancer patients has uncovered thousands of mutations that may associated with tumor growth, but scientists are uncertain about how most of these mutations actually contribute to cancer.
Cancer is caused by a buildup of several mutations within individual cells, but it is unclear how many of these mutations actually contribute to cancer. (Source: National Human Genome Research Institute)
For decades, researchers have used mice to study how these mutations cause cancer. By manually altering the DNA of mice to mimic the mutations found in human cancers, scientists can study the resulting effects in a controlled environment. These models have enabled researchers to observe the progression of cancer from its earliest stages and to test the efficacy of potential treatments. Mice models have been crucial for discovering how certain genes contribute to cancer and for testing new treatments, improving the way we treat cancer today.
However, mouse models have limitations. One major weakness is that mice can only model a fraction of the many thousands of possible mutations that cause cancer, so researchers might miss out on understanding some cancer types or mutations. This is due to several reasons: first, mice are typically bred to have a relatively uniform genetic background, which simplifies experimental conditions but limits the range of mutations that can be studied. This uniformity means that mouse models often represent only a subset of the genetic diversity found in human cancers. Secondly, while gene editing techniques have improved drastically over the past several decades, especially with the emergence of CRISPR-Cas9 technology, achieving absolute precision is still difficult. CRISPR-Cas9 mediated gene edited can have off-target effects, where unintended mutations occur elsewhere in the genome, can complicate results and lead to inaccurate representations of cancer processes. Moreover, while CRISPR–Cas9 can easily disrupt the activity of a gene, it cannot easily alter the activity of a gene. Given that over 80% of cancer mutations involve an alteration of a single part of a gene, CRISPR-Cas9 simply cannot install a majority of possible cancer mutations in mouse models.
Historically, mouse models have been used to study cancer mutations. However, scientists have failed to successfully induce a majority of the most common cancer mutations in mice (Dranoff, 2012).
To overcome the difficulties of traditional gene editing, scientists at the Massachusetts Institute of Technology were interested in using a new technique to model cancer mutations more effectively: prime editing. Prime editing improves upon existing CRISPR technology by combining two important enzymes: one to make a precise cut in the DNA and another to copy and insert the altered genetic information. Importantly, the cut occurs only one strand of the DNA, avoiding any double-stranded DNA breaks that can lead to errors during DNA repair. This method can create a wide range of genetic changes, including simple edits and more complex modifications. Remarkably, prime editing can mimic up to 96% of the cancer-related mutations found in human studies, and many of these can be matched to similar areas in the mouse genome, making mouse models more accurate and useful for studying cancer.
“This is a remarkably powerful tool for examining the effects of essentially any mutation of interest in an intact animal, and in a fraction of the time,” said Tyler Jacks, PhD, the David H. Koch Professor of Biology and researcher at the Koch Institute for Integrative Cancer Research at MIT.
This research was a collaboration between Jacks and David R. Liu, PhD, the Thomas Dudley Cabot Professor of the Natural Sciences at Harvard University and core institute member of the Broad Institute. While Jacks had built his career on developing genetically-engineered mouse models of cancer (GEMMs), it was Liu's team who engineered the prime editing technology that enabled more precise and versatile genetic modifications.
The labs of Tyler Jacks, PhD (left) and David R. Liu, PhD (right) joined forces to generate the novel genetically engineered mouse model with the ability to represent most of the various cancer alleles found in human tumors.
The teams joined forces to generate a new type of genetically-engineered mouse model that they called PE GEMM, which incorporates prime editing directly into the mice’s germline cells. In normal prime editing, the tools needed for the process—the prime editing guide RNA (pegRNA) and the prime editing enzyme (PE)—have to be delivered directly into cells. This is usually done through methods like injecting or using viral vectors. Instead of delivering these tools directly, the researchers opted to insert DNA that encodes these tools into the cells' genomes. This DNA integrates into the mouse cells' genome, allowing the cells to produce the editing tools themselves. This approach ensures that the prime editing tools are consistently present in all the cells that take up the DNA, which can improve the effectiveness of the editing and make it easier to study or use in therapy.
Not only did the researchers wish to ensure that the prime editing machinery was constantly present throughout the mouse, but they also wanted to control when and where this machinery was active in order to ensure precise edits. To set this up, the DNA sequence encoding the prime editing tools was engineered to be inactive under normal conditions. When Cre recombinase, an enzyme, is introduced into the cells, it rearranges the prime editing DNA sequence, thereby activating the production of the prime editing enzymes. This system allows the prime editing tools to remain inactive until triggered by Cre recombinase, providing precise control over when and where the editing occurs and helping to reduce the risk of off-target effects.
To illustrate their technology’s potential, the researchers turned their attention to the Kras gene, a key player in about 30% of human cancers, including nearly all pancreatic adenocarcinomas. Kras mutations, which often occur at the G12 position where glycine is located, vary widely. This site can be mutated to several different amino acids, leading to distinct cancer behaviors. The team focused on four specific Kras mutations linked to lung cancer: G12C, G12D, G12R, and G12A. They developed models for each mutation and observed surprising variability in tumor characteristics. While tumors with the G12R mutation turned out to be large and aggressive, those with the G12A mutation were smaller and progressed more slowly, highlighting how different mutations can dramatically affect cancer progression.
This innovative approach with prime editing marks a significant leap forward in genetic research, offering scientists a powerful tool to induce any desired mutations in mice with unprecedented precision. By embedding the prime editing system directly into the mouse genome, researchers can easily and accurately model specific genetic alterations, not just for cancer studies but for a wide range of genetic diseases and biological processes. As this technology continues to evolve, it holds the promise of transforming our approach to genetic research and precision medicine, ultimately leading to more targeted and effective treatments for a variety of diseases.
Original Article: https://doi.org/10.1038/s41587-023-01783-y